Childress A, Hayman K, Asubonteng K, Yarullina I, Earnest J, Rubin J.
Presented at American Association of Psychiatric Pharmacists (AAPP) 2024
An open-label, flexible-dose, safety and efficacy study of viloxazine ER in pediatric patients with ADHD experiencing suboptimal* efficacy to psychostimulants.

*IR-ADHD-RS-5 score ≥24 and a CGI-S score ≥3 (mildly ill or worse) at screening and baseline.
An open-label, flexible-dose, safety and efficacy study of viloxazine ER in pediatric patients with ADHD experiencing suboptimal* efficacy to psychostimulants.

*IR-ADHD-RS-5 score ≥24 and a CGI-S score ≥3 (mildly ill or worse) at screening and baseline.
PARTICIPANTS RECEIVED VILOXAZINE ER
(48/56)
PARTICIPANTS COMPLETED THE STUDY
(39/56)
MALE
(17/56)
FEMALE
CHILDREN
(AGED 6-11 YEARS)
ADOLESCENTS
(AGED 12-17 YEARS)
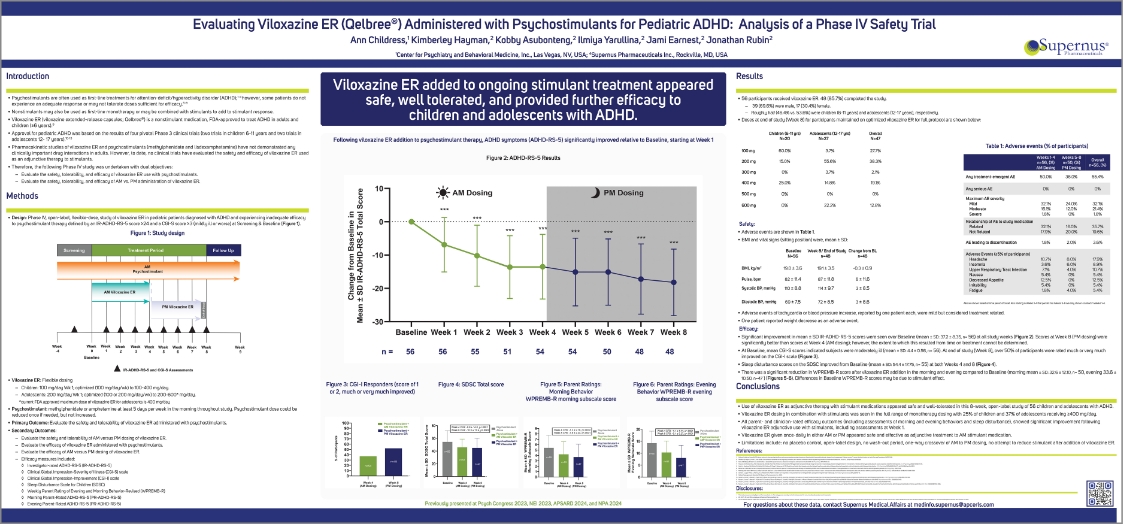
ADHD, attention-deficit/hyperactivity disorder; CGI-I, Clinical Global Impression-Improvement; CGI-S, Clinical Global Impression-Severity of Illness; ECG, electrocardiogram; ER, extended-release; FDA, US Food and Drug Administration; IR-ADHD-RS-5, Investigator-Rated Attention-Deficit/Hyperactivity Disorder-Rating Scale-5; SAE, serious adverse event; SDSC, Sleep Disturbance Scale for Children; TEAE, treatment-emergent adverse event.
PARTICIPANTS RECEIVED VILOXAZINE ER
(48/56) PARTICIPANTS COMPLETED THE STUDY
(39/56) MALE
(17/56) FEMALE
CHILDREN
(AGED 6-11 YEARS)
ADOLESCENTS
(AGED 12-17 YEARS)

ADHD, attention-deficit/hyperactivity disorder; CGI-I, Clinical Global Impression-Improvement; CGI-S, Clinical Global Impression-Severity of Illness; ECG, electrocardiogram; ER, extended-release; FDA, US Food and Drug Administration; IR-ADHD-RS-5, Investigator-Rated Attention-Deficit/Hyperactivity Disorder-Rating Scale-5; SAE, serious adverse event; SDSC, Sleep Disturbance Scale for Children; TEAE, treatment-emergent adverse event.